Contract Manufacturing of Oligonucleotide & Peptide APIs
Our Services
From Exploratory Research to Commercial Manufacturing
In the oligonucleotide and peptide API manufacturing business, we respond to customer needs in each stage, from small-volume synthesis for drug efficacy and toxicity evaluation to contract research on processing and method development for pilot manufacturing and GMP-compliant API manufacturing.

Services from the exploratory stage to the non-clinical stage are provided in the Pharmaceuticals Synthesis Laboratory, and services from the clinical stage to the commercial stage are provided in the GMP facility.
We have two manufacturing lines for the synthesis and purification of oligonucleotides, and one manufacturing line for peptides, which makes us one of the largest manufacturer of oligonucleotide and peptide APIs in Japan.
- Laboratory (non-GMP)
- CytivaÄKTA™ oligopilot plus 100
- CytivaÄKTA pure™ 150
- CytivaÄKTA™ avant 150
- Small-scale Oligonucleotide synthesizer
- Small-scale Peptide synthesizer
- Ultrafiltration equipment
- Lyophilizer
- GMP Facility
- Cytiva OligoPilot™ 400
- Peptide synthesizer (30L)
- Low pressure purification system Column diameter: 100㎜, 200mm
- High pressure purification system Column diameter: 200㎜, 450mm
- Ultrafiltration equipment
- Lyophilizer
- Quality control laboratory UHPLC
- Waters ACQUITY UPLC H-Class Bio
- Thermo Fisher Scientific UltiMate 3000
- Thermo Fisher Scientific Q Exactive
- Thermo Fisher Orbitrap Exploris MX
Off-line 2D-LC
High Resolution Mass Spectrometer (HRMS)
This Table includes trademarks of Cytiva, Waters, and Thermo Fisher.
-

Oligonucleotide API manufacturing
-
Peptide API manufacturing
Quality Assurance
We provide high quality GMP-compliant APIs.
GMP (Good Manufacturing Practice) is the standard for quality control and manufacturing control of pharmaceutical products.
We have established GMP-compliant SOPs (standard operation procedures) and provide education and training to all employees involved in our pharmaceutical manufacturing to ensure GMP standards.
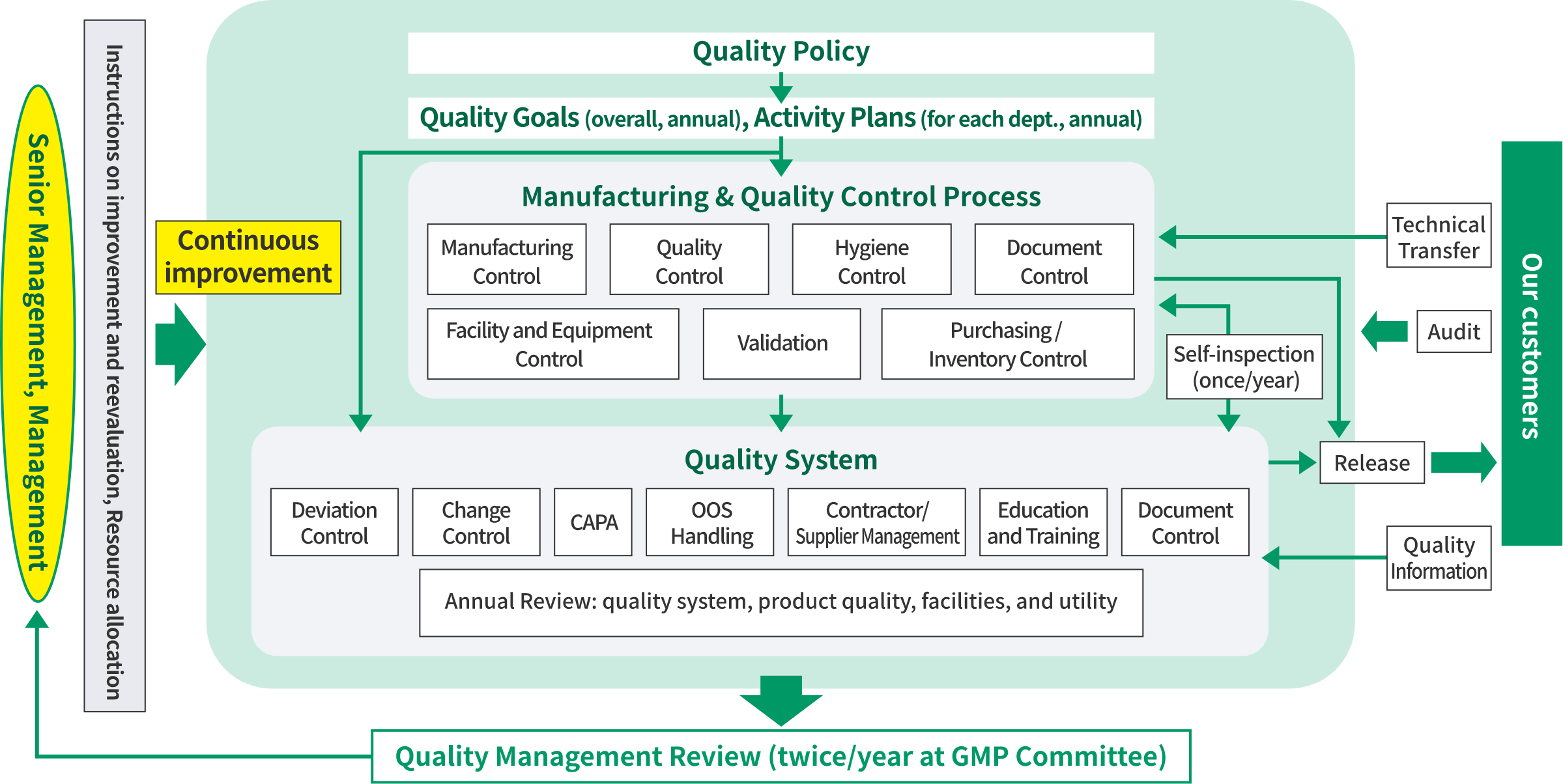
Quality Management System
We have implemented a quality management system required for pharmaceutical products and established our quality policy and quality goals. Based on the quality policy, we set quality goals for each fiscal year, and our GMP activities are reviewed by senior management in the GMP Committee twice a year (a management review) to ensure the continuous delivery of quality products.
- Quality Policy*
- ・We provide quality APIs for clinical trials to obtain the trust and satisfaction of our customers.
- ・We make continuous improvements to ensure the steady supply and higher quality of APIs for clinical trials.
- *We plan to obtain a pharmaceutical manufacturing license in the future.
- Our declaration on Data Integrity
- As a CDMO involved in drug development, one way we are committed to patients is by ensuring data integrity (completeness, consistency, and accuracy of data).
TOPICS
(1) Preventing cross-contamination
One of the requirements for a GMP facility is the prevention of contamination.
We have set three cleanliness levels for the manufacturing areas of our GMP facility, with ISO class 7 (during non-working time) being the highest.
To prevent unwanted mixing of products and contamination by foreign contaminants, the transfer routes of personnel, materials and equipment have been cafefully designed. For example, changing rooms and airlocks are properly installed for personnel, and pass-rooms are properly installed for materials and equipment. In addition, appropriately designed multiple air conditioning systems were installed according to manufacturing lines and cleanliness classes, so that the pressure difference between different cleanliness classes are properly maintained.
(2)Ensuring safety
Based on our company motto of “safety precedes production,” we conduct decomposition calorimetry, reaction heat measurements, and chemical engineering simulations to ensure safe manufacturing. We have also strictly implemented explosion-proof measures.


